Children's Hospital Neonatal Consortium
CDH Pain and Sedation Research Study sponsored by physicians at Colorado Children's Hospital and Lurie Children's Hospital of Chicago
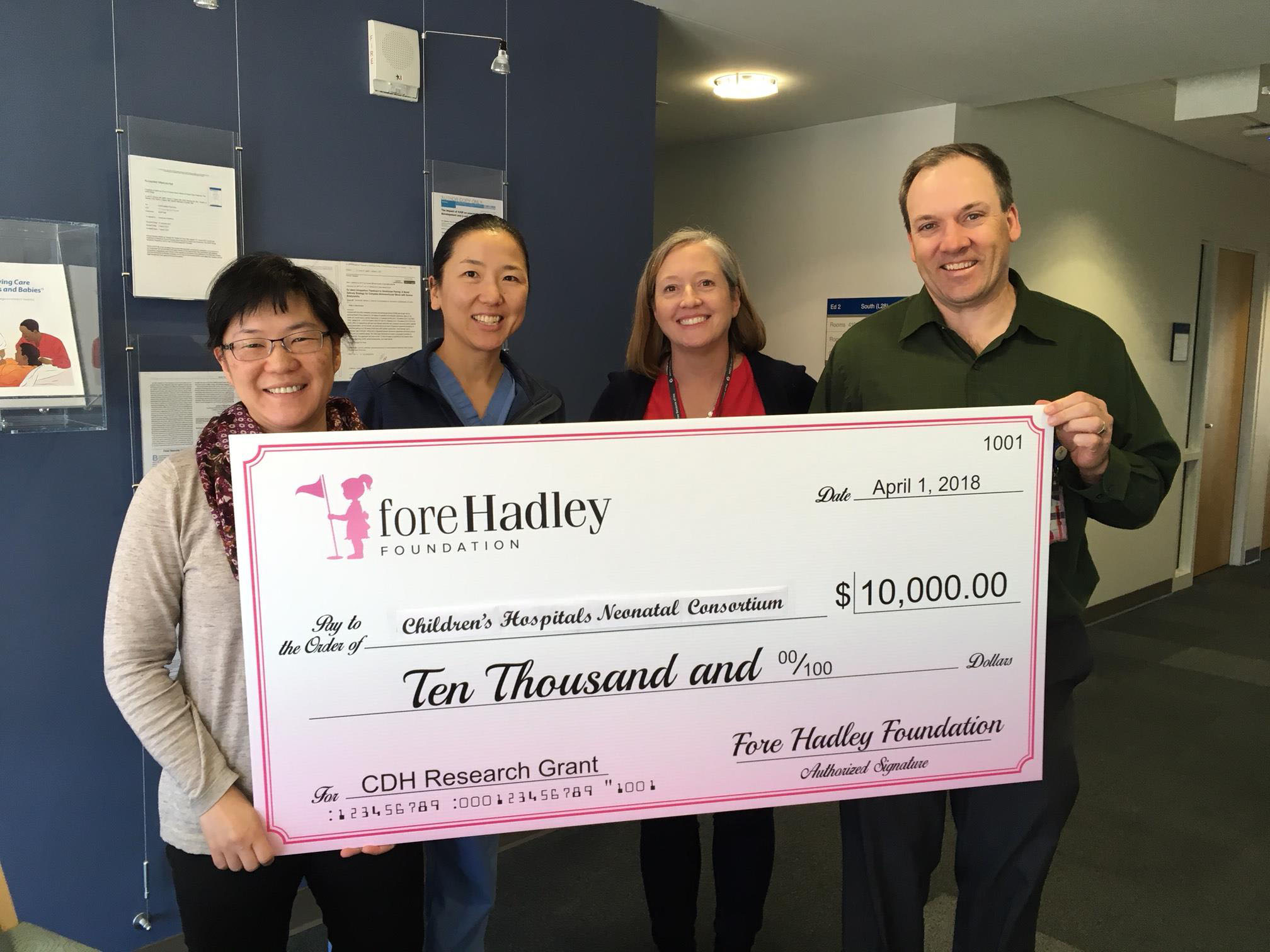
The Fore Hadley Foundation has partnered with the Children's Hospitals Neonatal Consortium (CHNC) by funding a research study aimed at improving pain management in infants with Congenital Diaphragmatic Hernia (CDH). CHNC is a group of 34 children’s hospitals and their neonatal intensive care units in the US and Canada that supports a focus group studying over 1,500 infants with CDH. The proposed study will examine use of pain and sedation medication use in CDH infants with a goal to understand and then optimize babies’ comfort during their hospitalization, while reducing side effects of these medications. The Fore Hadley Foundation and CHNC are excited to embark on this journey and anticipate that this partnership is the first of many collaborative efforts.
For infants with CDH, analgesic medications to relieve pain are essential after a surgical procedure(s). With pain, infants typically require more respiratory support, burn more energy, and clearly experience discomfort, and all of these decrease these infants’ ability to interact with their environment and sometimes their families. With prolonged support for breathing, like mechanical ventilation, infants with CDH frequently receive sedative medications to mask discomfort they may be experiencing as a result of their disease and/or therapies. In addition, infants with severe pulmonary hypertension typically require sedation to improve their clinical stability until the pulmonary hypertension improves. The short – and long– term effects of these medications on the developing brain are not well understood, and thus, clinicians must balance the need for analgesia and sedation in the short term with the possibilities of inducing long term consequences in surviving infants. Thus, there is a pressing need for better understanding of which medications offer clinical benefit with the fewest side effects.
The objectives of this grant will translate into a number of deliverables that would fundamentally represent new information for clinicians and families and a resource to assist clinicians and families to care for affected children:
- A verbal and/or slide presentation to the Fore Hadley Organization, (6) months from project initiation, describing the findings, their interpretation, and their implication on patient care
- Presentation at (2) national/international meetings for neonatologists to share our results and hear the implications of our findings
- A resultant peer-reviewed publication summarizing these same core concepts in a medical journal for the field/readers to gain understanding what questions were answered, and what those answers mean
- Lay the foundation for the next improvement project: to develop a comfort – oxygenation score for providers and parents to jointly assess the well-being of their child upon which real-time clinical interventions can be validated and based
- Develop evidence-based and science-based clinical guidelines for pain and sedation management in infants with CDH, to be published and shared with medical providers throughout the world